Investigational FMT Treatments and Eligibility
Investigational fecal microbiota transplantation (FMT) is a promising therapy for patients with recurrent C. difficile infections. Interested in learning more? The information below will help determine if you’re eligible for an investigational FMT.

What is Investigational FMT?
Investigational FMT is a therapy that is not approved by the FDA and must be accessed under an approved research protocol. Currently, you must consult your doctor about whether an investigational FMT is an appropriate option for you.
Questions about Investigational FMT?
Navigating access to investigational medical treatments isn’t always easy. OpenBiome is here to help. If you’re having trouble finding answers or simply prefer to talk with someone one-on-one, reach out to us at the provided email address or phone number.
Contact the OpenBiome Outreach Team
Introduction to Investigational FMT
Within all of us is a community of bacteria known as the microbiome. These bacteria help our bodies to digest food, fight infections, and perform other key functions that keep us healthy.
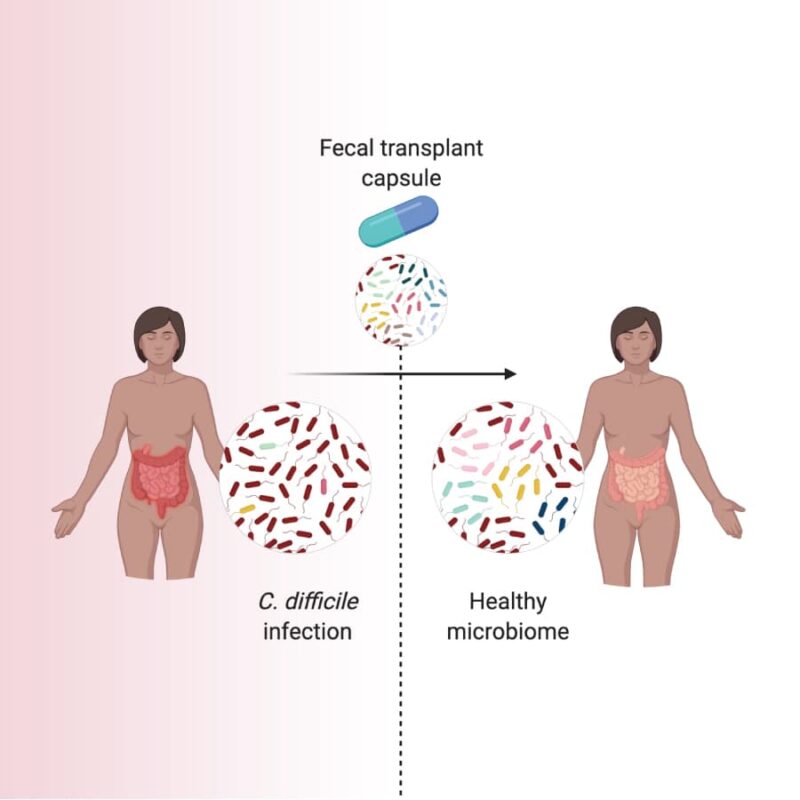
Investigational fecal microbiota transplantation (FMT) is a medical procedure that transfers the microbial community from a screened, healthy donor into a patient’s gut to help treat their illness. This procedure is typically performed through a colonoscopy.
Using Investigational FMT to Treat C. difficile Infections
Most patients with C. difficile are infected after receiving antibiotics for an unrelated illness.
Although antibiotics are powerful and life-saving medications, they may also disrupt the healthy bacteria that normally prevents the growth of infectious microbes like C. difficile.
Some studies suggest that investigational FMT may repopulate a patient’s damaged microbiome with a diverse community of microorganisms. This community may limit C. difficile's ability to find the space and nutrients to grow.
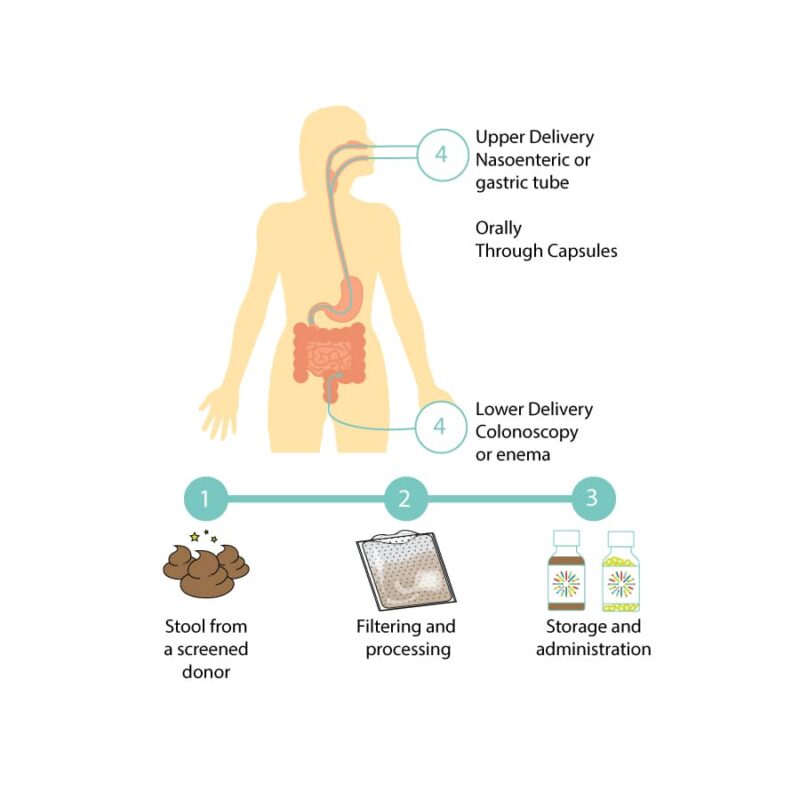
What Goes into Making and Receiving an Investigational FMT Preparation?
OpenBiome's collaborator, the University of Minnesota, follows the steps below to manufacture investigational FMT preparations.
- Donor screening and stool donation: A donor who has passed screening for potential health risks provides a stool sample.
- Processing: The stool sample is mixed with a buffer to help bacteria survive being frozen. The resulting mixture is filtered to remove fiber and solid particles that may clog the machinery used to administer the investigational FMT treatment.
- Storage and Administration: Newly made investigational FMT preparations are stored frozen in a -80 degree Celsius freezer. Preparations are shipped overnight on dry ice to hospitals where they are once again stored in freezers or thawed and administered to a patient.
Patients, depending on their unique health status and medical history, may receive investigational FMT in three different modalities.
- Upper delivery (though a nasoenteric/gastric tube or EGD)
- Lower delivery (though colonoscopy, sigmoidoscopy, or enema)
- Orally (through a capsule form)
Investigational FMT is typically an outpatient procedure, and patients are usually able to return home a few hours after the procedure.
Am I Eligible for an Investigational FMT?
It’s important to speak with your doctor about whether investigational FMT is the right option for you. Patients who meet the requirements below may be eligible for an investigational FMT treatment
1. Diagnosis of C. difficile infection (CDI).
a. You have been diagnosed with mild or moderate CDI and have failed to respond to at least two courses of standard-of-care therapies.
OR
b. You have been diagnosed with severe/fulminant CDI that has failed standard-of-care therapy
2. Informed consent Conversation: You have discussed investigational FMT as a treatment option with your doctor and understand
a. That investigational FMT is an investigational treatment that is not approved by the FDA.
b. The potential risks of investigational FMT as well as alternative treatment options, including to receive no treatment.
Next Steps for Receiving an Investigational FMT
If you are eligible for an investigational FMT, the next step is to further discuss the treatment with your doctor.
Once you make a decision together, your doctor will place an order with OpenBiome. Once ordered, it typically takes a few days for an investigational FMT treatment to arrive at the hospital where your procedure will take place.
Additional Resources About Investigational FMT
We’ve curated the following resources on investigational FMT and C. difficile to provide a more comprehensive understanding of what’s been discussed above. This information is for physicians and patients alike.
Selection from OpenBiome’s White Paper Collection
We Are Here To Help
The OpenBiome Outreach Team is always happy to answer your questions over the phone or email. We also provide more information in our Patient FAQ.