We provide therapies, microbes, data, and knowledge to fill critical gaps in microbiome research and health care.
Research Programs
Our programs accelerate microbiome science— ethically and equitably— to improve health worldwide.
Investigational FMT Access Program
OpenBiome provides access to investigational fecal transplants as a last line of defense for patients with antibiotic-resistant C. difficile infections.
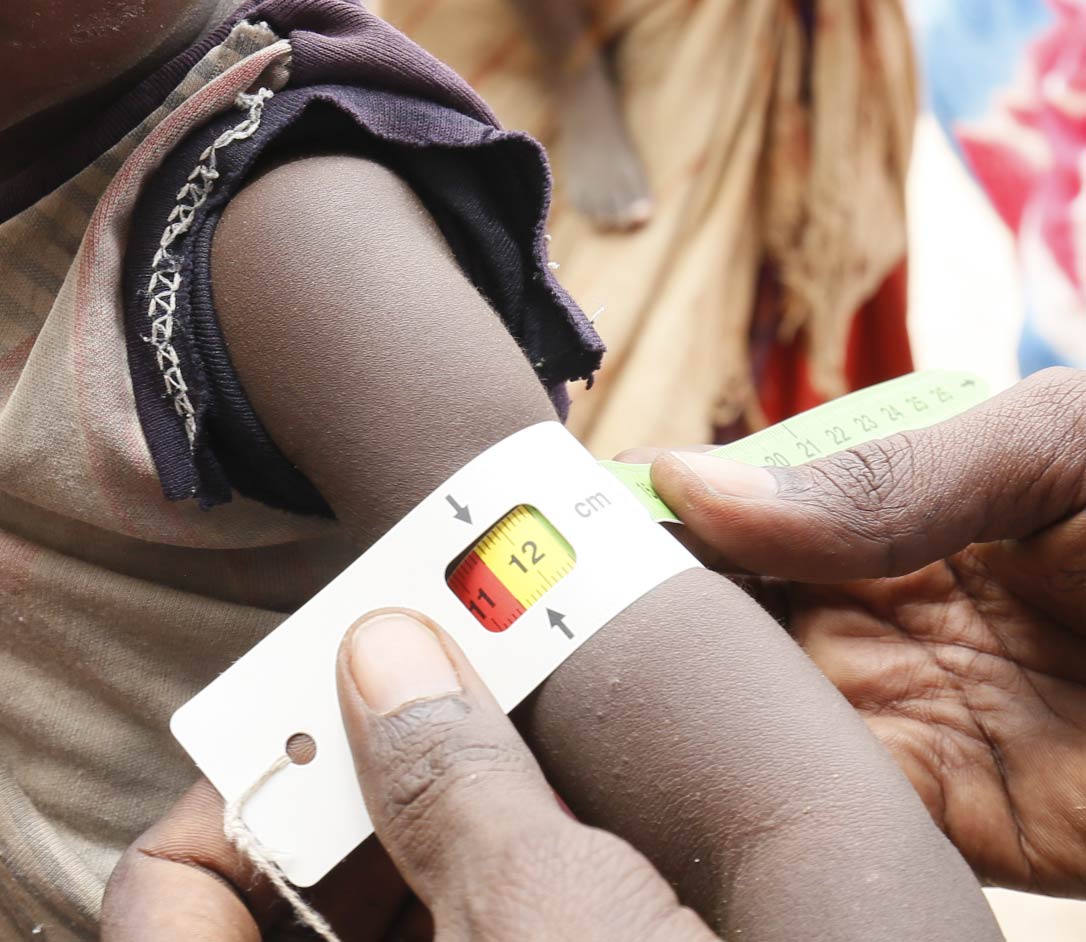
Malnutrition Program
OpenBiome and our collaborators are developing live biotherapeutic therapies to help treat nearly 200 million children worldwide affected by malnutrition.
Our Impact
Over the past 10 years, we've pushed scientific boundaries and set gold standards
Patients have received urgently needed investigational fecal microbiota transplantation (FMT) treatments distributed by OpenBiome.
Clinical trials and single patient case studies have used OpenBiome investigational FMT to explore the microbiome as a treatment target for a wide range of indications.
Latest News and Spotlights
As Featured In
OpenBiome has appeared in national and international publications. Read more coverage here.